Table of Contents
ToggleWhat is quantum mechanics?
Quantum mechanics is for the most part viewed as the physical hypothesis that is our best contender for a major and general depiction of the actual world. The applied system utilized by this hypothesis contrasts radically from that of old-style physical science. For sure, the change from traditional to quantum physical science denotes genuine revolution in our comprehension of the physics.
The uncertainty principle is positively perhaps the most celebrated and significant parts of quantum mechanics. It has regularly been viewed as the most particular element in which quantum mechanics varies from classical hypotheses of the actual world. Generally, the uncertainty rule expresses that one can’t allocate accurate synchronous qualities to the position and force of a physical framework. Maybe, these amounts must be resolved with some trademark uncertainties’ that can’t turn out to be subjectively little all the while. In any case, what is the specific importance of this principle, and in fact, is it actually a rule of quantum mechanics? And, specifically, what’s the significance here to say that an amount is resolved simply up to some vulnerability? These are the primary inquiries exploring in the following, focusing on the perspectives on Heisenberg and Bohr.
Heisenberg Uncertainty Principle
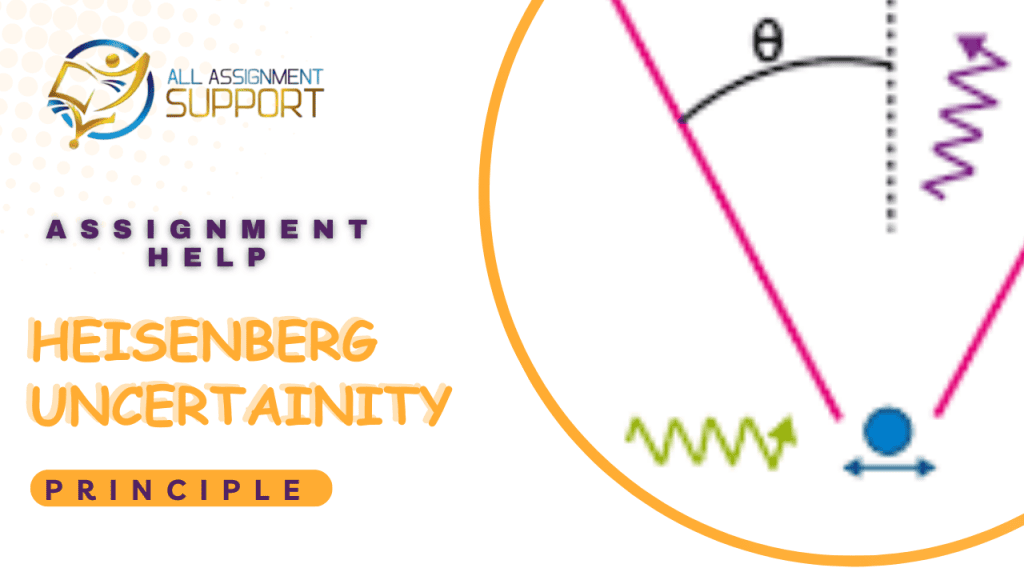
The uncertainty principle likewise called the Heisenberg Uncertainty Principle, or Indeterminacy Principle, verbalized on 1927 by the German physicist Werner Heisenberg, that the position and the speed of an item can’t both be estimated precisely, simultaneously, even in principle. The actual ideas of precise position and definite velocity together, truth be told, have no significance in nature.
Common experience gives no insight of this guideline. It is not difficult to quantify both the position and the speed of, say, a vehicle, in light of the fact that the vulnerabilities suggested by this standard for conventional articles are too little to possibly be noticed. The total principle specifies that the result of the uncertainties in position and speed is equivalent to or more prominent than a small actual amount, or steady around 10-34 joule-second, the estimation of the amount h, where h is Planck’s consistent. Just for the tiny masses of molecules and subatomic particles does the result of the uncertainties become critical.
Any endeavor to quantify definitely the speed of a subatomic molecule, like an electron, will thump it about in a capricious way, so a synchronous estimation of its position has no legitimacy. This outcome has nothing to do with deficiencies in the estimating instruments, the strategy, or the spectator; it emerges out of the close association in nature among particles and waves in the domain of subatomic measurements.
Each molecule has a wave related with it; every molecule really shows wavelike conduct. The molecule is destined to be found in where the undulations of the wave are most prominent, or generally serious. The more extraordinary the undulations of the related wave become, however, the more not well characterized turns into the frequency, which thus decides the force of the molecule. So, a stringently limited wave has an uncertain frequency; its related molecule, while having an unequivocal position, has no specific speed. A molecule wave having a very much characterized frequency, then again, is fanned out; the related molecule, while having a fairly exact speed, might be anyplace. A very precise estimation of one noticeable includes a moderately huge uncertainty in the estimation of the other.
The vulnerability rule is on the other hand communicated as far as a molecule’s force and position. The energy of a molecule is equivalent to the result of its mass occasions its speed. Accordingly, the result of the uncertainties in the force and the situation of a molecule approaches h/2. The standard applies to other conjugated sets of observables, like energy and time: the result of the uncertainty in an energy estimation and the uncertainty in the time span during which the estimation is made likewise approaches h/2. A similar connection holds, for a precarious molecule or core, between the vulnerability in the amount of energy emanated and the vulnerability in the lifetime of the temperamental framework as it makes a change to a steadier state.
The principle revolves around the three particles Time, Position, Energy/Velocity. A trademark highlight of quantum material science is the principle of complementarity, which “infers the inconceivability of any sharp partition between the conduct of nuclear items and the cooperation with the estimating instruments which serve to characterize the conditions under which the marvels show up.” therefore, “proof acquired under various trial conditions can’t be fathomed inside a solitary picture, yet should be viewed as reciprocal as in just the entirety of the wonders depletes the conceivable data about the articles.” This understanding of the significance of quantum physical science, which suggested an adjusted perspective on the importance of actual clarification, steadily came to be acknowledged by most of physicists during the 1930’s.
Read Also : Physics Assignment Help
Numerically we portray the vulnerability standard as the accompanying, where ‘x’ is position and ‘p’ is energy: the numerical type of the vulnerability standard relates correlative to Planck’s steady information isn’t limitless, implicit indeterminacy exists, however just in the tiny world, all implodes to determinism in the perceptible world. This is maybe the most well-known condition close to E=mc2 in material science. It fundamentally says that the blend of the mistake in position times the blunder in energy should consistently be more noteworthy than Planck’s steady. In this way, you can gauge the situation of an electron to some exactness, however then its energy will be inside a huge scope of qualities. Similarly, you can quantify the energy accurately, yet then its position is obscure. Notice that this isn’t the estimation issue in another structure, the mix of position, energy and time are really unclear for a quantum molecule until an estimation is made at that point the wave work breakdowns. Likewise notice that the vulnerability guideline is irrelevant to perceptible items since Planck’s consistent, h, is so little (10-34). For instance, the vulnerability in position of a tossed baseball is 10-30 millimeters.
The profundity of the vulnerability guideline is acknowledged when we pose the inquiry; is our insight into reality limitless? The appropriate response is no, in light of the fact that the vulnerability standard expresses that there is an underlying vulnerability, indeterminacy, geocentricism to Nature. Understandably, Heisenberg was miserable about this turn of events. In a letter of 8 June 1926 to Pauli he admitted that “The more Factual piece of Schrödinger’s hypothesis, the seriously nauseating I discover it”, and: “What Schrodinger expounds on the Anschaulichkeit of his hypothesis, bout Mist Pauli, 1979, p. 328″.
Once more, this last German term is deciphered distinctively by different reporters: as “garbage” Miller, 1982 “junk” Beller 1999, poop” Cassidy, 1992, and maybe more in a real sense, as “bologna” (de Regt, 1997). All things considered, in distributed compositions, Heisenberg voiced a more adjusted assessment. In a paper in Die Naturwissenschaften, 1926 he summed up the curious circumstance that the synchronous advancement of two contending speculations had achieved. Despite the fact that he contended that Schrödinger’s understanding was indefensible, he conceded that grid mechanics didn’t give the Anschaulichkeit which made wave mechanics so appealing. He finished up: “to acquire a logical inconsistency free anschaulich understanding, we actually come up short on some fundamental element in our picture of the design of issue”. The reason for his 1927 paper was to give precisely this lacking element.
Need help ? Contact All Assignment Support, We are Online.